Current location:
Default>Services
>Production process validation service
工藝驗(yàn)證流程
CREATE TIME:2018-06-29 11:36BROWSE TIMES:3650
為保證穩(wěn)定地生產(chǎn)合格產(chǎn)品�����,投產(chǎn)前對(duì)產(chǎn)品生產(chǎn)系統(tǒng)所進(jìn)行的驗(yàn)證工作����。它的內(nèi)容是:根據(jù)設(shè)計(jì)質(zhì)量的要求,利用生產(chǎn)系統(tǒng)影響產(chǎn)品質(zhì)量的規(guī)律性��,通過(guò)試生產(chǎn)�����,對(duì)設(shè)備、原材料和零配件���、工藝和操作規(guī)程以及生產(chǎn)環(huán)境等進(jìn)行分析����,確認(rèn)生產(chǎn)系統(tǒng)所生產(chǎn)的產(chǎn)品能達(dá)到質(zhì)量要求��,以保證投產(chǎn)后產(chǎn)品能夠穩(wěn)定地符合各項(xiàng)質(zhì)量要求���。
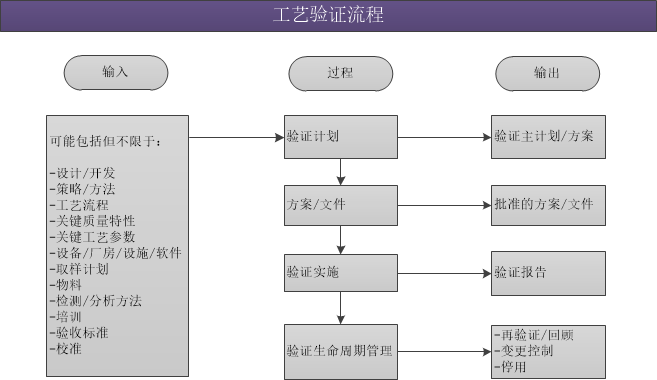
工藝驗(yàn)證:從工藝設(shè)計(jì)階段到商業(yè)生產(chǎn)的整個(gè)過(guò)程中��,對(duì)數(shù)據(jù)進(jìn)行收集和評(píng)價(jià)����,建立工藝能始終如一地交付出優(yōu)質(zhì)產(chǎn)品中的科學(xué)證據(jù)�����。工藝驗(yàn)證涉及整個(gè)產(chǎn)品生命周期和生產(chǎn)中發(fā)生的一系列活動(dòng)��。包括3階段:
第一階段 - 工藝設(shè)計(jì):在開(kāi)發(fā)和放大活動(dòng)過(guò)程中獲得的知識(shí)基礎(chǔ)上����,在此階段對(duì)商業(yè)化生產(chǎn)工藝進(jìn)行定義�。
第二階段 - 工藝確認(rèn):在此階段�,對(duì)工藝設(shè)計(jì)進(jìn)行評(píng)估,以確認(rèn)工藝是否具備可重現(xiàn)的商品化制造能力�����。
第三階段 - 持續(xù)工藝確認(rèn):在日常生產(chǎn)中獲得工藝處于受控狀態(tài)的持續(xù)的保證�。
PREVIOUS: NOTHING
NEXT: NOTHING